Miscellaneous
Hi! Here you'll find a fun/useful general science snippet and a physics note, followed by some brief information about the spelling of my name.
(Click here for past snippets & notes).
Science snippet #6: Caring for a new book
I recently encountered a trick to condition a new book to prevent the spine from cracking. This was sadly too late for one book (note the orange book below), but saved another (see the blue book). A cracked spine can cause pages to fall out, but if the spine bends instead of cracks then all is well. In mathematical terms we want the slope of the spine to remain continuous.


The process takes about ten minutes (less than the time spent reading a book!), and adopts the following approach:
-- Start with the book at room temperature or warmer, and before opening the book fully.
-- The general strategy is to place the book with its spine on a desk and gently push along the inside of the spine, with the spine kept concave as seen from below (check out the videos here and here).
-- I usually start near the middle of the book, with half the pages lying flat on the desk and the other half held about 45° above the horizonal. I then lift around five pages each time up from the horizontal, pushing along the inside of the spine until all the pages originally lying flat have been lifted.
-- I then repeat this for the other half of the book.
Now, enjoy!
Physics note #7: Some musing about low-E windows (at the upper-undergrad level)
While recently replacing a double-glazed pane in a window, I was curious both what is meant by a "low-E" coating, and how to determine which of the four possible surfaces of a double-glazed window has this coating on it.
I'll explore in this note the first of these, and move to the second at a later date.
The short answer is that a low-E (low-emissivity) coating has two effects. First it helps the glass reflect, rather than absorb, incident mid-infrared wavelengths. This 'thermal' region of the electromagnetic spectrum is that at which objects with temperatures from –30 to +40 °C generally emit, and so the coating improves the insulation effects of a window by reducing heat flow through radiation. Additionally these coatings can be engineered to either increase or decrease the reflectance of near-IR & UV wavelengths from the Sun while leaving visible transmission relatively unscathed, which changes the amount of energy from sunlight that passes through the window, and thus further reduces either heating or cooling costs.
This topic at hand is 'The optical properties of solids', and we are interested here in glass a few millimeters thick, and metal several nanometers thick. Check out the first plot below, which shows the refractive index for silica, and which would be similar for most clear and uncoated glass. Shown are the two parts of the complex refractive index: the real part nR (simply called the “refractive index”, which we often encounter when exploring refraction), and the imaginary part nI (the “extinction coefficient”, which indicates how steeply light intensity decreases as light travels through a medium). Together, nR and nI determine the reflectance of a surface, and thus how much light enters the medium, and nI together with the medium's thickness determines how much of this light is subsequently absorbed. The plot displays wavelengths from 20 µm (photon energy of 0.62 eV) to 125 nm (energy of 9.92 eV), where strong absorption due to having passed over the band gap energy at ~9.2 eV is already very visible.
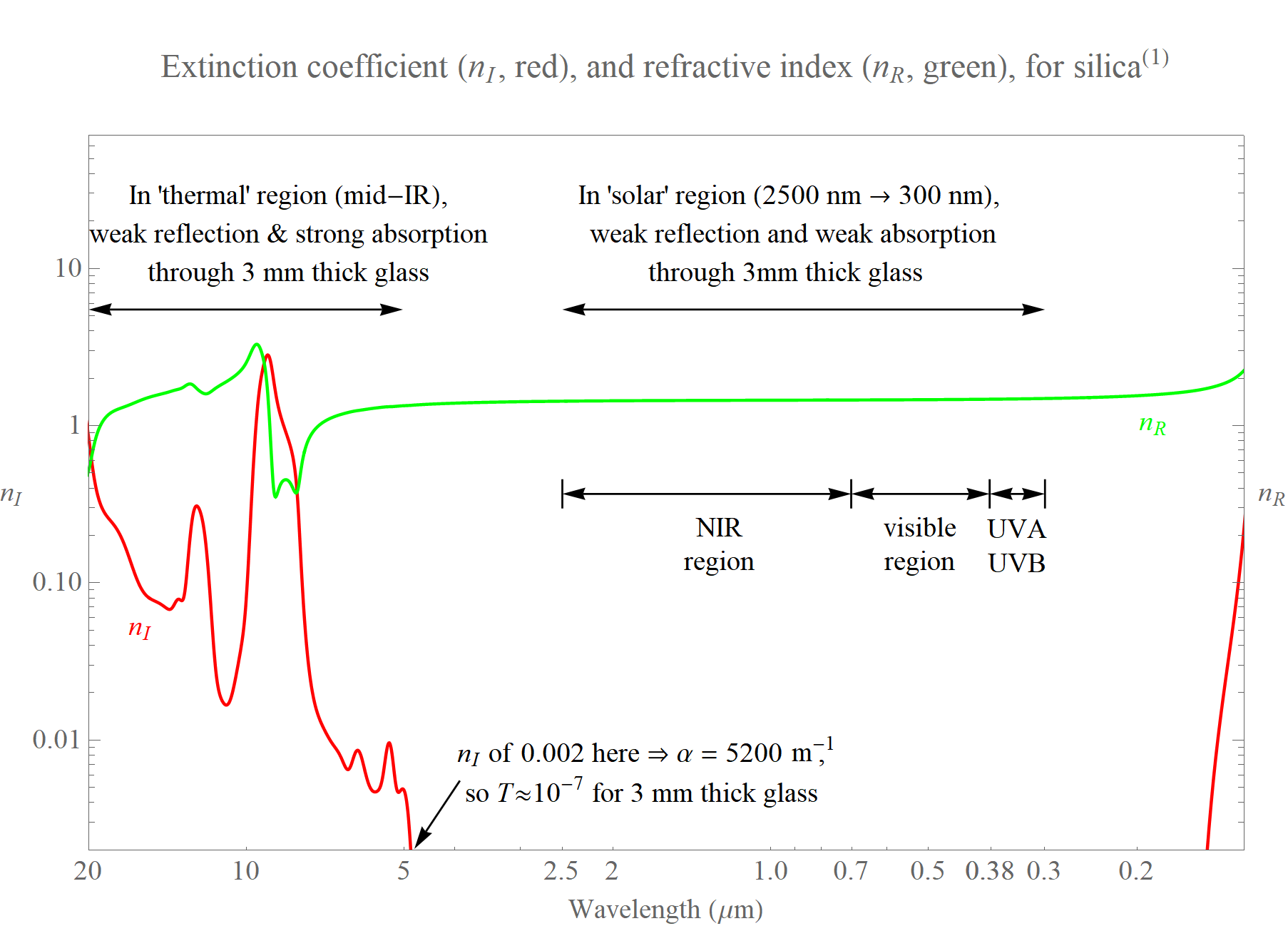
For wavelengths from 5–20 µm (in the 'thermal' region of the spectrum) the refractive index is between 0.4 and about 3, and the extinction coefficient between 0.002 and 3. These values of nI and nR result in a relatively low reflectance of uncoated glass at these wavelengths, so most incoming mid-IR radiation enters the glass. However, even for an extinction coefficient of 0.002 at a wavelength of 5 µm, the absorption coefficient, α, is more than 5000 m-1, which for 3 mm of glass means strong absorption and very low transmittance (~10-7).
Heat flow generally occurs through a mix of conduction, convection, and radiation. Compared to a single pane of glass, a double-pane made from two pieces of clear, uncoated, glass exhibits much-reduced conduction and convection. However, as indicated by the previous paragraph, much of the thermal radiation that strikes the pane on the hotter side of the window is absorbed, which heats up the pane, which then radiates heat towards the other pane. Therefore a double-glazed pane made from uncoated glass does not strongly reduce heat transfer by radiation.
Specifications of various types of uncoated and coated glasses used in windows across North America are held by the International Glazing Database (IGDB). From this we learn that a single 3-mm-thick uncoated clear glass pane has transmittance, reflectance, and absorption values for the thermal region of around 0%, 16%, and 84% respectively. (These values account for multiple reflections, and apply to near-normal incidence of incoherent light). Thus ~84% of the incident thermal radiation is absorbed by this glass, and subsequently re-emitted - it has high emissivity.
Meanwhile, in the ‘solar’ region (from 300 -2500 nm) the extinction coefficient is small; and in fact it is reduced by a factor of ~1010 compared to values in the mid-IR region. The 3 mm thick glass just considered has transmittance, reflectance, and absorption for the solar region of 83%, 8%, and 9%, respectively, and for the narrower visible region (defined as 380–700 nm) these are 90%, 9%, and 1%, respectively.
High solar transmittance can significantly heat a dwelling from incoming sunlight, which can help on cold days but is a hindrance on hot days. The window industry quantifies this heating effect, including secondary heating due to absorption of sunlight followed by conduction/convection through a window, by the solar heat gain coefficient (SHGC), which adopts a value between 0 and 1. A double-pane window made from two uncoated glass panes has an SHGC value of typically 0.75 or more.
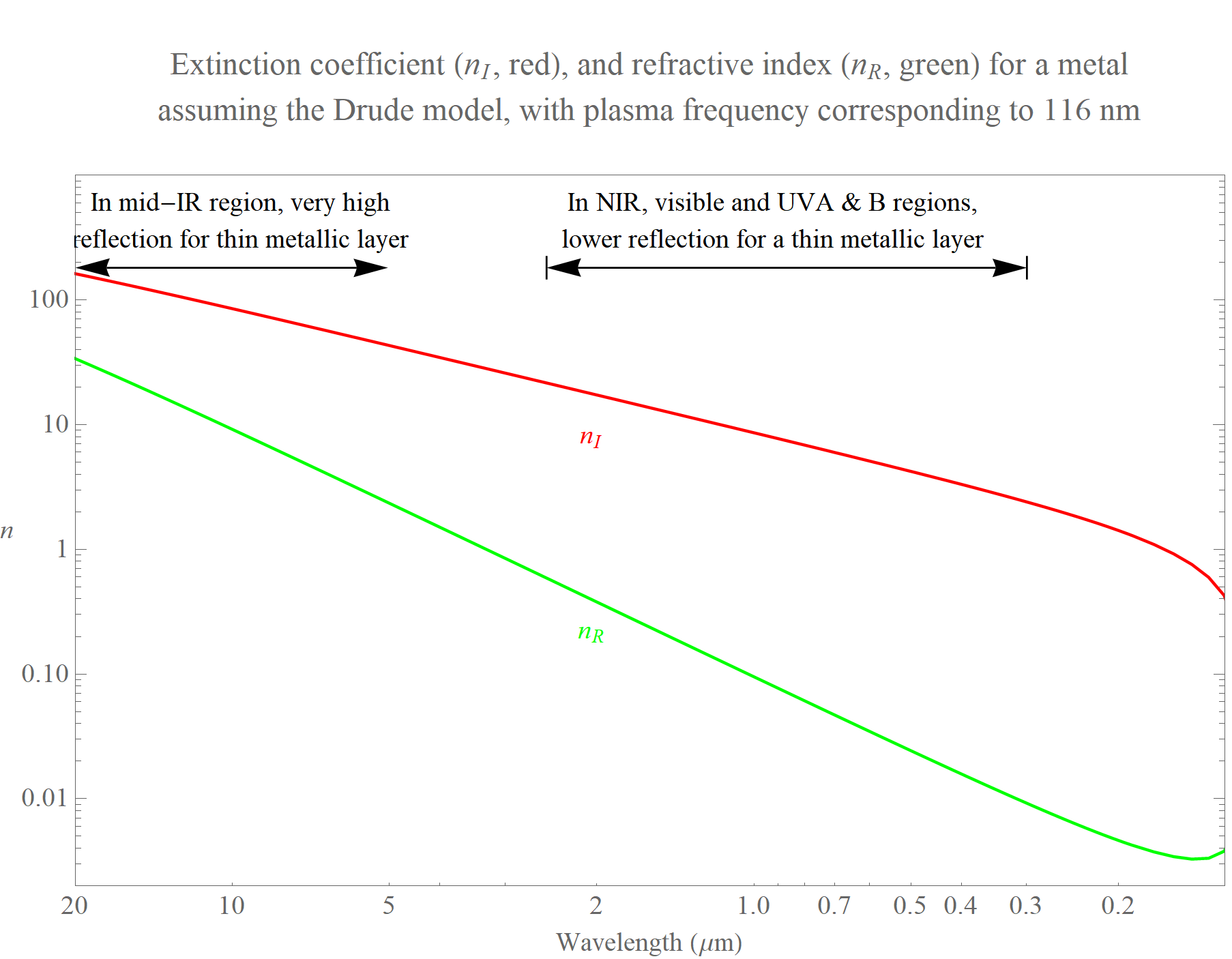
Now check out the complex refractive index of a metal, for which we shall adopt a Drude model with a plasma frequency corresponding to a wavelength of 116 nm (so a photon energy of 10.69 eV, just off the right side of the graph). In the mid-IR region nR and nI are so large that the reflectance of the metal is very high, and this reflectance is substantially higher than in the visible region. However, for very thin layers of the metal there is little absorption of light that does enter the material. Thus, glass coated with a very thin layer of, say, silver will exhibit the desired low-E behavior for thermal (mid-IR) radiation.(2) A particular coated glass in the database - #5142 - has transmittance, reflectance, and absorption for the thermal region of 0%, 90%, and 10%, respectively. The large mid-IR reflectance compared to that of uncoated glass means that this glass strongly reduces heat flow due to radiation.
We also see that the reflectance of the metal across the NIR region will be greater than for visible light, and so a window with a metal-coated surface would have a reduced SHGC compared to uncoated glass with little effect on visible light transmission. Indeed, by using various coatings it is possible to selectively and specifically increase the reflectance in the NIR and the UV regions. For glass #5142 the solar-region transmittance, reflectance, and absorption values are 57%, 26%, and 17%, while in the visible region they are 83%, 5%, and 12%. In addition to reducing heat flow through thermal radiation, such a glass also reduces the SHGC value, which further reduces cooling costs on hot sunny days.
This illustrates one of the many real-world applications of optics, and of understanding the optical properties of materials. If you are interested in learning more, check out our new textbook, Pedrottis' Introduction to Optics, 4th ed.
(1) D. Franta et al, "Optical characterization of SiO2 thin films using universal dispersion model over wide spectral range", Proc. SPIE, 989014 (2016).
(2) Metal films retain their high reflectance even for thicknesses well below the skin depth, and only when they are a few nanometers thick or less do they then exhibit how reflectance. For more information see A. E. Kaplan, "Metallic nanolayers: a sub-visible wonderland of optical properties", JOSAB, 35, 1328 (2018).
Finally, a note about 'Rayf'
I usually adopt the spelling Rayf, which is consistent with the pronunciation I prefer (/reɪf/), and used for many years by family and friends. The original (and still the formal, legal) spelling of my name is Ralph, yet this can be confusing to all in a similar way to the Stroop effect. Some information about the origin of the name, from Prof. Ralph Wedgewood at the University of Southern California, can be found here.
